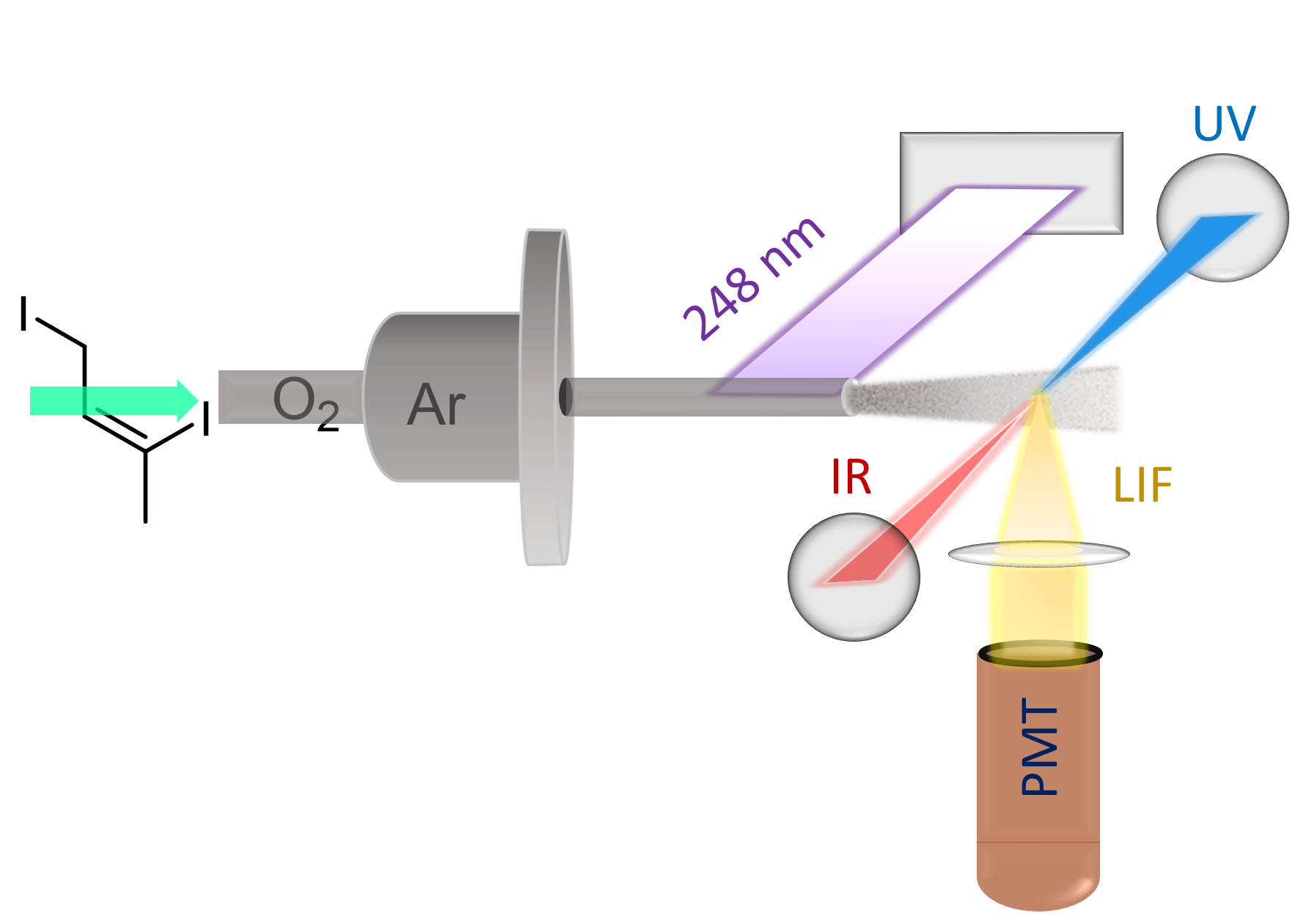
We use IR action spectroscopy to study the unimolecular decay dynamics of atmospheric reaction intermediates. Photolytically generated monoiodo radicals react with molecular oxygen in order to generate Criegee intermediates. The Criegee intermediates are then cooled in a supersonic expansion. An IR pump is used to vibrationally activate these reactive intermediates with a well-defined amount of energy, which allows the Criegee intermediates to surmount or tunnel through the transition state barrier and produce OH radical products. The resulting OH radicals are detected via UV laser induced fluorescence (LIF).
Schematic of the IR action spectroscopy experimental setup
Two types of experiments are possible using this technique. In the first, the IR wavelength is scanned at a fixed time delay to generate an IR action spectrum. In the second, the IR wavelength is fixed on a known IR transition of the Criegee intermediate, and the IR-UV time delay is varied. This technique allows us to measure the energy-dependent OH appearance rate from Criegee intermediates in real time.
This technique has been applied to various alkyl-substituted Criegee intermediates, and has allowed us to validate the 1,4 hydrogen-atom transfer mechanism by which OH is formed from unimolecular decay of Criegee intermediates. In particular, it has shown that quantum mechanical tunneling is extremely important in this reaction mechanism.
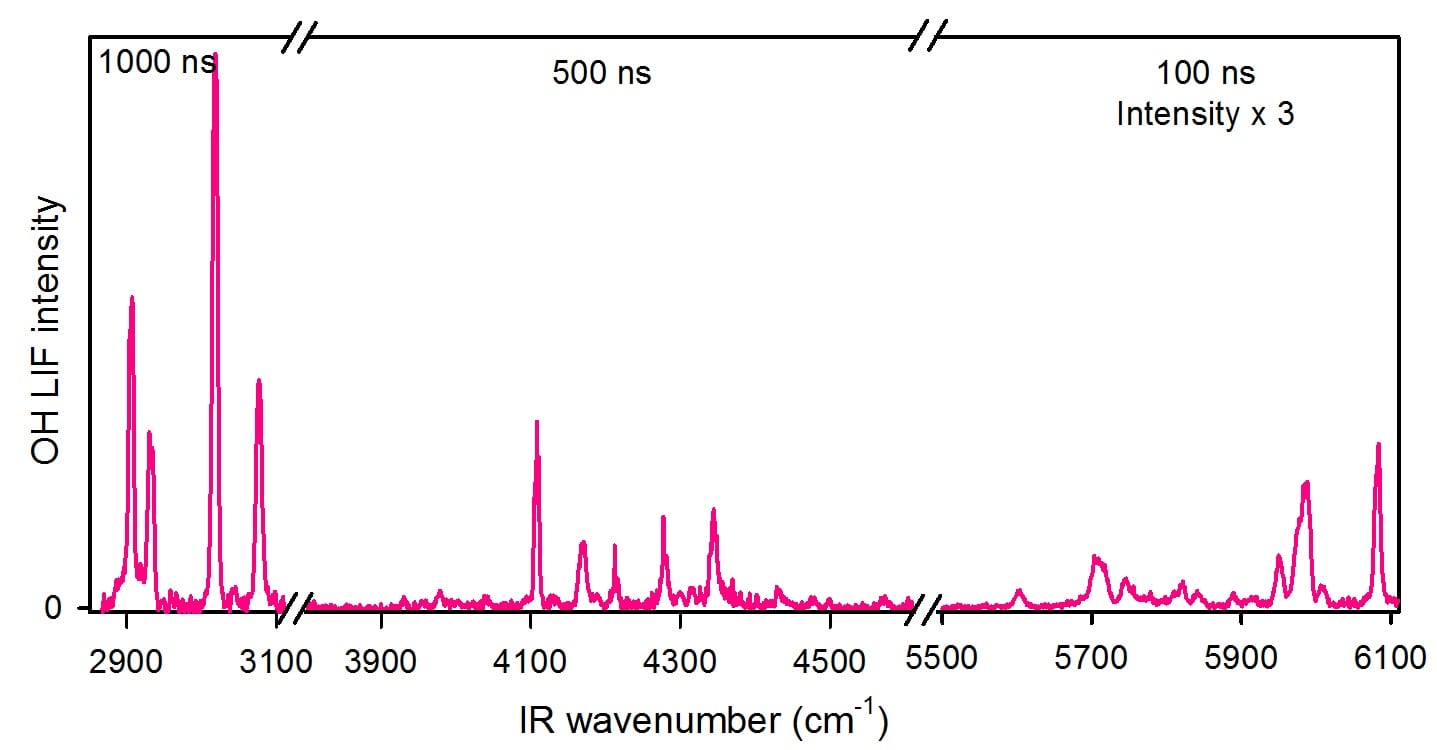 IR action spectrum of syn-CH3CHOO following IR activation in 3 different energy regimes, and at 3 different time delays
IR action spectrum of syn-CH3CHOO following IR activation in 3 different energy regimes, and at 3 different time delays
Most recently, we have applied this technique to achieve the first detection of the methyl vinyl ketone oxide (MVK-oxide) Criegee intermediate, which is the predominant four carbon Criegee intermediate resulting from isoprene ozonolysis.
Schematic for the IR experiment for MVK-oxide