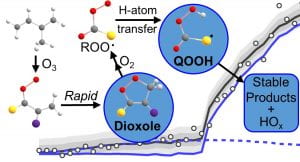
Hydroperoxyalkyl Radical
(•QOOH) IR Spectroscopy and
Unimolecular Dissociation
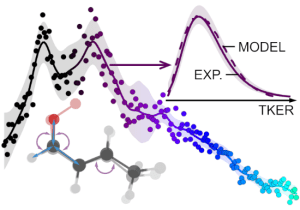
T. Bhagde, A. S. Hansen, S. Chen, P. J. Walsh, S. J. Klippenstein, and M. I. Lester, “Energy-resolved and time-dependent unimolecular dissociation of hydroperoxyalkyl radicals (•QOOH)”, Faraday Discuss. 238, 575 – 588 (2022). https://doi.org/10.1039/D2FD00008C
A. S. Hansen, T. Bhagde, Y. Qian, A. Cavazos, R. M. Huchmala, M. A. Boyer, C. F. Gavin-Hanner, S. J. Klippenstein, A. B. McCoy, and M. I. Lester, “Infrared Spectroscopic Signature of a Hydroperoxyalkyl Radical (•QOOH)”, J. Chem. Phys. 156, 014301 (2022). PDF.
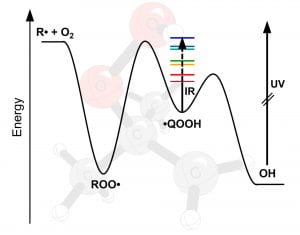
A. S. Hansen, T. Bhagde, K. B. Moore III, D. R. Moberg, A. W. Jasper, Y. Georgievskii, M. F. Vansco, S. J. Klippenstein, and M. I. Lester, “Watching a Hydroperoxyalkyl Radical (•QOOH) Dissociate”, Science 373, 679-682 (2021). PDF

VUV Photoionization and
TOF Mass Spectrometry
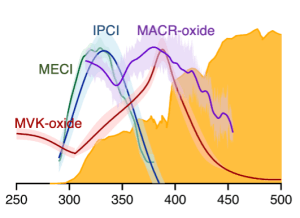
G. Wang, T. Liu, M. Zou, C. A. Sojdak, M. C. Kozlowski, T. Karsili, and M. I. Lester, “Electronic Spectroscopy and Dissociation Dynamics of Vinyl-Substituted Criegee Intermediates: 2-Butenal Oxide and Comparison with Methyl Vinyl Ketone Oxide and Methacrolein Oxide Isomers”, J. Phys. Chem. A 127, 203–215 (2022). https://doi.org/10.1021/acs.jpca.2c08025
 T. Liu, M. Zou, A. Caracciolo, C. A. Sojdak, and M. I. Lester, “Substituent effects on the electronic spectroscopy of four-carbon Criegee intermediates”, J. Phys. Chem. A 126, 6734-6741 (2022). https://doi.org/10.1021/acs.jpca.2c05502
T. Liu, M. Zou, A. Caracciolo, C. A. Sojdak, and M. I. Lester, “Substituent effects on the electronic spectroscopy of four-carbon Criegee intermediates”, J. Phys. Chem. A 126, 6734-6741 (2022). https://doi.org/10.1021/acs.jpca.2c05502
M. F. Vansco, M. Zou, I. O. Antonov, K. Ramasesha, B. Rotavera, D. L. Osborn, Y. Georgievski, C. J. Percival, S. J. Klippenstein, C. A. Taatjes, M. I. Lester, and R. L. Caravan, “Dramatic conformer-dependent reactivity of the acetaldehyde oxide Criegee intermediate with dimethylamine via a 1,2-insertion mechanism”, J. Phys. Chem. A 126, 710–719 (2022). PDF.
M. F. Vansco, K. Zuraski, F. A. F. Winiberg, K. Au, N. Trongsiriwat, P. J. Walsh, D. L. Osborn, C. J. Percival, S. J. Klippenstein, C. A. Taatjes, M. I. Lester, and R. L. Caravan, “Functionalized Hydroperoxide Formation from the Reaction of Methacrolein-oxide, an Isoprene-Derived Criegee Intermediate, with Formic Acid: Experiment and Theory”, Molecules 26, 3058 (2021). PDF

R. L. Caravan, M. F. Vansco, and M. I. Lester, “Open questions on the reactivity of Criegee intermediates”, Commun. Chem.4, 44 (2021). PDF
M. F. Vansco, R. L. Caravan, S. Pandit, K. Zuraski, F. A. F. Winiberg, K. Au, T. Bhagde, N. Trongsiriwat, P. J. Walsh, D. L. Osborn, C. J. Percival, S. J. Klippenstein, C. A. Taatjes, and M. I. Lester, “Formic Acid Catalyzed Isomerization and Adduct Formation of an Isoprene-Derived Criegee Intermediate: Experiment and Theory”, Phys. Chem. Chem. Phys. 22, 26796-26805 (2020). PDF

M. F. Vansco, R. L. Caravan, K. Zuraski, F. A. F. Winiberg, K. Au, N. Trongsiriwat, P. J. Walsh, D. L. Osborn, C. J. Percival, M. A. H. Khan, D. E. Shallcross, C. A. Taatjes, and M. I. Lester, “Experimental evidence of dioxole unimolecular decay pathway for isoprene-derived Criegee intermediates”, J. Phys. Chem. A 124, 3542-3554 (2020). PDF
R. L. Caravan, M. F. Vansco, K. Au, M. A. H. Khan, Y.-L. Li, F. A. F. Winiberg, K. Zuraski, Y.-H. Lin, W. Chao, N. Trongsiriwat, P. J. Walsh, D. L. Osborn, C. J. Percival, J. Jr-M. Lin, D. E. Shallcross, L. Sheps, S. J. Klippenstein, C. A. Taatjes, and M. I. Lester, “Direct kinetic measurements and theoretical predictions of an isoprene-derived Criegee intermediate”, Proc. Natl. Acad. Sci. 117, 9733-9740 (2020). PDF
M. F. Vansco, B. Marchetti, N. Trongsiriwat, G. Wang, T. Bhagde, P. J. Walsh, S. J. Klippenstein, and M. I. Lester, “Synthesis, electronic spectroscopy and photochemistry of methacrolein oxide: A four carbon unsaturated Criegee intermediate from isoprene ozonolysis”, J. Am. Chem. Soc. 141, 15058−15069 (2019). PDF
M. F. Vansco, B. M. Marchetti, and M.I. Lester, “Electronic Spectroscopy of Methyl Vinyl Ketone Oxide: A Four-Carbon Criegee Intermediate from Isoprene Ozonolysis.” J. Chem. Phys. 149, 244309 (2018). PDF
C. A. Taatjes, F. Liu, B. Rotavera, M. Kumar, R. Caravan, D. L. Osborn, W. H. Thompson, and M. I. Lester, “Hydroxyacetone production from C3 Criegee intermediates”, J. Phys. Chem. A 121, 16-23 (2017). PDF
A. M. Green, F. Liu, and M. I. Lester, “UV + VUV double-resonance studies of autoionizing Rydberg states of the hydroxyl radical”, J. Chem. Phys.144, 184311 (2016). PDF
F. Liu, Y. Fang, M. Kumar, W. H. Thompson, and M. I. Lester, “Direct observation of vinyl hydroperoxide”, Phys. Chem. Chem. Phys. (Communication) 17, 20490-4 (2015). PDF
J. M. Beames, F. Liu, and M. I. Lester, “1+1′ resonant ionization of OH radicals via the A2Σ+ state: Insights from direct comparison with A-X fluorescence detection”, Mol. Phys. 112, 897-903 (2014). PDF
F. Liu, J. M. Beames, A. M. Green, and M. I. Lester, “UV spectroscopic characterization of dimethyl- and ethyl-substituted carbonyl oxides”, J. Phys. Chem. A 118, 2298–2306 (2014). PDF
J. M. Beames, F. Liu, L. Lu, and M. I. Lester, “UV spectroscopic characterization of an alkyl substituted Criegee intermediate CH3CHOO”, J. Chem. Phys. 138, 244307 (2013). PDF
J. M. Beames, F. Liu, L. Lu, and M. I. Lester, “Ultraviolet spectrum and photochemistry of the simplest Criegee intermediate CH2OO”, J. Am. Chem. Soc. (Communication) 134, 20045-48 (2012). PDF
J. M. Beames, F. Liu, M. I. Lester, and C. Murray, “Communication: A new spectroscopic window on hydroxyl radicals using UV+VUV resonant ionization”, J. Chem. Phys. 134, 241102 (2011). PDF
Velocity Map Imaging
G. Wang, T. Liu, M. Zou, T. Karsili, and M. I. Lester, “UV Photodissociation Dynamics of the Acetone Oxide Criegee Intermediate: Experiment and Theory”, Phys. Chem. Chem Phys. 25, 7453–7465 (2023). https://doi.org/10.1039/D3CP00207A
G. Wang, T. Liu, A. Caracciolo, M. F. Vansco, N. Trongsiriwat, P. J. Walsh, B. Marchetti, T. Karsili, and M. I. Lester, “Photodissociation Dynamics of Methyl Vinyl Ketone Oxide: A four-carbon unsaturated Criegee intermediate from isoprene ozonolysis”, J. Chem. Phys. 155, 174305 (2021). PDF.
V. J. Esposito, T. Liu, G. Wang, A. Caracciolo, M. F. Vansco, B. Marchetti, T. Karsili, and M. I. Lester, “Photodissociation Dynamics of CH2OO on Multiple Potential Energy Surfaces: Experiment and Theory”, J. Phys. Chem. A 125, 30, 6571–6579 (2021). PDF.
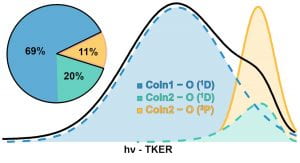
M. F. Vansco, H. Li, and M. I. Lester, “Prompt release of O 1D products upon UV excitation of CH2OO Criegee intermediates”, J. Chem. Phys. 147, 013907 (2017). PDF
H. Li, N. M. Kidwell, X. Wang, J. M. Bowman, and M. I. Lester, “Velocity map imaging of OH radical products from IR activated (CH3)2COO Criegee intermediates”, J. Chem. Phys. 145 104307 (2016). PDF
N. M. Kidwell, H. Li, X. Wang, J. M. Bowman, and M. I. Lester, “Unimolecular dissociation dynamics of vibrationally activated CH3CHOO Criegee intermediates to OH radical products”, Nat. Chem. 8, 509-14 (2016). PDF
H. Li, Y. Fang, N. M. Kidwell, J. M. Beames, and M. I. Lester, “UV photodissociation dynamics of the CH3CHOO Criegee intermediate: Action spectroscopy and velocity map imaging of O-atom products”, J. Phys. Chem. A. 119, 8328-37 (2015). PDF
H. Li, Y. Fang, J. M. Beames, and M. I. Lester, “Velocity map imaging of O-atom products from UV photodissociation of the CH2OO Criegee intermediate”, J. Chem. Phys. 142, 214312 (2015). PDF
J. H. Lehman, H. Li, J. M. Beames and M. I. Lester, “Communication: Ultraviolet photodissociation dynamics of the simplest Criegee intermediate CH2OO”, J. Chem. Phys. 139, 141103 (2013). PDF
IR Action Spectroscopy and
Unimolecular Decay Dynamics
A. S. Hansen, Y. Qian, C. A. Sojdak, M. C. Kozlowski, V. J. Esposito, J. S. Francisco, S. J. Klippenstein, and M. I. Lester, “Rapid allylic 1,6 H-atom transfer in an unsaturated Criegee intermediate”, J. Am. Chem. Soc. 144, 5945–5955 (2022). PDF.
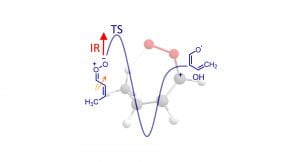
A. S. Hansen, R. Huchmala, E. Vogt, M. Boyer, T. Bhagde, M. F. Vansco, C. V. Jensen, A. Kjærsgaard, H. G. Kjærgaard, A. B. McCoy, and M. I. Lester, “Coupling of Torsion and OH-Stretching in tert-Butyl Hydroperoxide 1: The Cold and Warm First OH-Stretching Overtone Spectrum”, J. Chem. Phys. 154, 164306 (2021). PDF
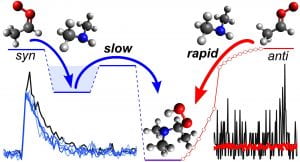
A. S. Hansen, Z. Liu, S. Chen, M. G. Schumer, P. J. Walsh, and M. I. Lester, “Unraveling conformer specific sources of hydroxyl radical production from an isoprene-derived Criegee intermediate by deuteration” J. Phys. Chem. A. 124, 4929–4938 (2020). PDF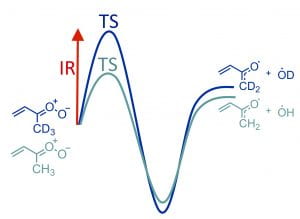
V. P. Barber, V. J. Esposito, T. Trabelsi, A. S. Hansen, T. A. McHenry, J. S. Francisco, and M. I. Lester, “Experimental and Computational Investigation of Vinoxy and 1-Methylvinoxy Radicals from the Unimolecular Decay of Alkyl-Substituted Criegee Intermediates”, Chem. Phys. Lett. 751, 137478 (2020). PDF
V. P. Barber*, A. S. Hansen*, Y. Georgievskii, S. J. Klippenstein, and M. I. Lester, “Experimental and theoretical studies of the doubly-substituted methyl-ethyl Criegee intermediate: Infrared action spectroscopy and unimolecular decay to OH radical products” J. Chem. Phys. 152, 094301 (2020). PDF (*equal contributions)
T. A. Stephenson and M. I. Lester, “Unimolecular decay dynamics of Criegee intermediates: Energy-resolved rates, thermal rates, and their atmospheric impact”, Int. Rev. Phys. Chem. 39, 1-33 (2020). PDF
V. P. Barber, S. Pandit, V. J. Esposito, A. B. McCoy, and M. I. Lester, “CH Stretch Activation of CH3CHOO: Deep Tunneling to Hydroxyl Radical Products”, J. Phys. Chem. A, 123, 2559-2569 (2019). PDF
V. P. Barber, S. Pandit, A. M. Green, N. Trongsiriwat, P. J. Walsh, S. J. Klippenstein, and M. I. Lester, “Four-Carbon Criegee Intermediate from Isoprene Ozonolysis: Methyl Vinyl Ketone Oxide Synthesis, Infrared Spectrum, and OH Production”, J. Am. Chem. Soc., 140, 10866-10880 (2018). PDF
M. I. Lester and S. J. Klippenstein, “Unimolecular decay of Criegee intermediates to OH radical products: Prompt and thermal decay processes”, Acc. Chem. Res. 51, 978-985 (2018). PDF
A. M. Green, V. P. Barber, Y. Fang, S. J. Klippenstein, and M. I. Lester, “Selective deuteration illuminates the importance of tunneling in the unimolecular decay of Criegee intermediates to hydroxyl radical products”, PNAS 114, 12372–7 (2017). PDF
G. T. Drozd, N. M. Donahue, T. Kurtén, and M. I. Lester, “Unimolecular decay of the dimethyl substituted Criegee intermediate in alkene ozonolysis: Decay timescales and the importance of tunneling”, J. Phys. Chem. A 121, 6036–6045 (2017). PDF
Y. Fang, V. P. Barber, S. J. Klippenstein, A. B. McCoy, and M. I. Lester, “Tunneling effects in the unimolecular decay of (CH3)2COO Criegee intermediates to OH radical products”, J. Chem. Phys 146, 134307 (2017). PDF
Y. Fang, F. Liu, V. P. Barber, S. J. Klippenstein, A. B. McCoy, and M. I. Lester, “Deep tunneling in the unimolecular decay of CH3CHOO Criegee intermediates to OH radical products”, J. Chem. Phys. 145, 234308 (2016). PDF
Y. Fang, F. Liu, S. J. Klippenstein, and M. I. Lester, “Direct observation of unimolecular decay of CH3CH2CHOO Criegee intermediates to OH radical products”, J. Chem. Phys. 145, 044312 (2016). PDF
Y. Fang, F. Liu, V. P. Barber, S. J. Klippenstein, A. B. McCoy, and M. I. Lester, “Communication: Real time observation of unimolecular decay of Criegee intermediates to OH radical products”, J. Chem. Phys. 144, 061101 (2016). PDF
F. Liu, J. M. Beames, and M. I. Lester, “Direct production of OH radicals upon CH overtone activation of (CH3)2COO Criegee intermediates”, J. Chem. Phys. 141, 234312 (2014). PDF
F. Liu, J. M. Beames, A. S. Petit, A. B. McCoy, and M. I. Lester, “Infrared-driven unimolecular reaction of CH3CHOO Criegee intermediates to OH radical products”, Science 345, 1596-1598 (2014). PDF