Neuropeptide Receptors – Hirschmann/Nicolaou/Smith Collaboration (1987-2011)

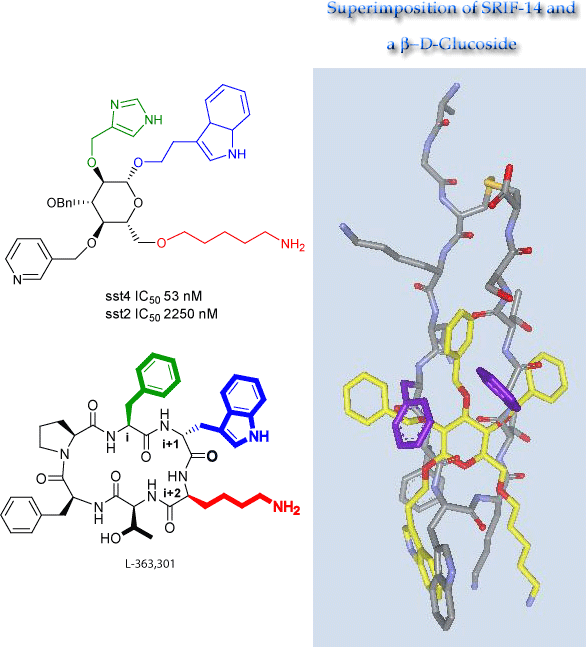
In collaboration with Professor Ralph Hirschmann (also a member of this department) we have designed and synthesized glucopyransides and found them to be mimics of the cyclic hexapeptide L-363,301 in that they bind to the SRIF receptor, mimicking the β-turn of the latter. We also discovered that these two compounds bind also structurally diverse proteins which we attribute to the radial symmetry features of the glucose scaffold.
Subsequent studies have produced several noteworthy findings: (1) the glycoside inhibits GRF-induced growth hormone (GH) release by cultured rat anterior pituitary cells with an IC50 of 3 micromolar (i.e. 1b is recognized at this endocrine receptor and it provides a sufficiently good fit to activate the receptor); (2) N-acetylation of the primary amino group generated an amide which was highly selective for the substance P (SP) receptor, with an IC50 of 60 nM. That such minor chemical modification should produce such a dramatic switch in biological profile was a pleasing but unexpected result. It is worth noting that SRIF receptors, as well as the SP, and the β2-adrenergic receptors, utilize G-protein-mediated signal transduction, suggesting that the binding of the glucosides to these three receptors may involve similar interactions within the conserved hydrophobic domains of the receptors.
Subsequently, analog synthesis generated an analog with an IC50 at the human SRIF receptor subtype 4 of 53 nM and another congener with an IC50 of 3 nM at the NK1 receptor. The glycosides have a binding profile at the SRIF receptor similar to analogously modified peptides. This demonstrates that the indole ethyl and 6-amino pentyl sidechains mimic the Trp and Lys residues, respectively, of the β-turn of L-363,301.
Unexpectedly a des-indole analog in which the C1 Trp-mimicking sidechain was replaced by a C1 methoxyl proved to be an SRIF agonist, a result which we see as but one example of the fact that the β-D-glucose scaffold possesses radial symmetry which confers other noteworthy properties on this scaffold. That the glucoside, but not L-363,301, binds the NK-1 receptor is also explained by the presence of radial symmetry in the former, but not in the c-hexapeptide. Interestingly, replacement of Lys9 of either L-363,301 or of D-Trp8-SRIF by p-F-Phe is required to change peptidal SRIF agonists into substance P antagonists. It is a consequence of the radial symmetry of the sugar scaffold that the same glucoside binds the SRIF receptor via the C-1 and C-6 sidechains but the NK-1 receptor via the C-1 and C-2 sidechains. Conversely, L-363,301 and its p-F-Phe9 analog both bind their respective receptors (SRIF and SP, respectively) via their i + 1 and i + 2 positions. In the course of this work we discovered that both indole and benzyl side chains, but not heterocycle side chains bind the Trp8 binding pocket. In collaboration with Professor E.R. Thornton we explained these results through correlation with the electrostatic potential of the aromatic rings.
Recently, we proposed that the small molecule binding domain of GPCRs ideally accommodates the sidechain projections of β-turns or other similarly oriented and exposed functionalities. From this perspective, the scaffolds become the counterpart to the privileged nature of their receptors.
Referenced Publications:
R. Hirschmann, K. C. Nicolaou, S. Pietranico, J. Salvino, E. M. Leahy, P. A. Sprengeler, G. Furst, A. B. Smith, III, C. D. Strader, M. A. Cascieri, M. R. Candelore, C. Donaldson, W. Vale, and L. Maechler. Nonpeptidal Peptidomimetics with a β-D-Gluco
R. Hirschmann, P. A. Sprengeler, T. Kawasaki, J. W. Leahy, W. C. Shakespeare, and A. B. Smith, III. The First Design and Synthesis of a Steroidal Peptidomimetic. The Potential Value of Peptidomimetics in Elucidating the Bioactive Conformation of Pe
R. Hirschmann, P. A. Sprengeler, T. Kawasakil J. W. Leahy, W. C. Shakespeare, and A. B. Smith, III. The Versatile Steroid Nucleus: Design and Synthesis of Peptidomimetic Employing this Novel Scaffold. Tetrahedron Symposium in Print 1993, 49, 3665-3
A. Lender, W. Yao, R. A. Spanevello, G. Furst, P. A. Sprengeler, R. Hirschmann, and A. B. Smith, III. Design and synthesis of sulfur-free cyclic hexapeptides which contain the RGD sequence and bind to the fibrinogen GP IIb/IIIa receptor. Int. J. Pe
R. Hirschmann, K. C. Nicolaou, S. Pietranico, E. M. Leahy, J. Salvino, B. Arison, M. A. Cichy, P. G. Spoors, W. C. Shakespeare, P. A. Sprengeler, P. Hamley, A. B. Smith, III, T. Reisine, K. Raynor, L. Maechler, C. Donaldson, W. Vale, R. M. Freidinger
Hirschmann, R.; Smith, A. B.; Sprengeler, P. A. Some interactions of macromolecules with low molecular weight ligands. Recent advances in peptidomimetic research. New Perspectives in Drug Design, [based on the Proceedings of the International Round Table
Hirschmann, Ralph; Yao, Wenqing; Cascieri, Margaret A.; Strader, Catherine D.; Maechler, Laurie; Cichy-Knight, Maria A.; Hynes, John, Jr.; van Rijn, Rachel D.; Sprengeler, Paul A.; Smith, Amos B., III. Synthesis of Potent Cyclic Hexapeptide NK-1 Antagonis
Hirschmann, Ralph; Yao, Wenqing; Arison, Byron; Maechler, Laurie; Rosegay, Avery; Sprengeler, Paul A.; Smith, Amos B., III. The first synthesis of a tricyclic homodetic peptide employing coordinated orthogonal protection. Tetrahedron Letters (1996), 37(32
Hirschmann, Ralph; Hynes, John, Jr.; Cichy-Knight, Maria A.; van Rijn, Rachel D.; Sprengeler, Paul A.; Spoors, P. Grant; Shakespeare, William C.; Pietranico-Cole, Sherrie; Barbosa, Joseph; Liu, Josephine; Yao, Wenqing; Rohrer, Susan; Smith, Amos B., III.
Hirschmann, Ralph; Yao, Wenqing; Arison, Byron; Maechler, Laurie; Rosegay, Avery; Sprengeler, Paul A.; Smith, Amos B., III. Synthesis of the first tricyclic homodetic peptide. use of coordinated orthogonal deprotection to achieve directed ring closure. Te
P. W. Schiller, I. Berezowska, T. M.-D. Nguyen, R. Schmidt, C. Lemieux, N. N. Chung, M. L. Falcone-Hindley, W. Yao, J. Liu, S. Iwama, A. B. Smith, III, and R. Hirschmann. Novel Ligands Lacking a Positive Charge for the δ and μ-Opioid Receptors. J.
J. Liu, D. J. Underwood. M. A. Cascieri, S. P. Rohrer, L.-D. Cantin, G. Chicchi, A. B. Smith, III and R. Hirschmann. Synthesis of a Substance P Antagonist with Somatostatin Scaffold: Factors Affecting Agonism/Antagonism at GPCRs and the Role of Pseu
R. Hirschmann, L. Ducry, and A. B. Smith, III. The Development of an Efficient Regio- and Stereoselective Route to Libraries Based on the β-D-Glucose Scaffold. J. Org. Chem. 2000, 65, 8307-8316.
L. Abrous, J. Hynes, Jr., S. R. Friedrich, A. B. Smith, III, and R. Hirschmann. Design and Synthesis of Novel Scaffolds for Drug Discovery: Hybrids of β-D-Glucose with 1,2,3,4 Tetrahydrobenzo[e][1,4]diazepin-5-one, the Corresponding 1-Oxazepinel and
A. B. Smith, III, L.-D. Cantin, A. Pasternak, L. Guise-Zawacki, W. Yao, A. Charnleyl J. Barbosa, P. A. Sprengeler, R. Hirschmann, S. Munshi, D. B. Olsen, W. A. Schleif and L. C. Kuo. Design, Synthesis and Biological Evaluation of Monopyrrolinone-Bas
A. B. Smith, III, Y. S. Cho, G. R. Pettit, and R. Hirschmann. Design, synthesis, and evaluation of azepine-based cryptophycin mimetics. Tetrahedron 2003, 59, 6991-7009.
L. Abrous, P. Jokiel, S. R. Friedrich, J. Hynes, Jr., A. B. Smith, III, and R. Hirschmann. Novel Chimeric Scaffolds to Extend the Exploration of Receptor Space: Hybrid β-D-Glucose-Benzoheterodiazepine Structures for Broad Screening. Effect of Amide
S. Neelamkavil, B. P. Mowery, E. R. Thornton, A. B. Smith, III, and R. Hirschmann. A Practical Synthesis of Nα-Fmoc-L-Pyrazinylalanine via Schöllkopf’s Chiral Auxiliary. J. Peptide Res. 2005, 65, 139-142.
A. R. Angeles, I. Neagu, E. T. Birzin, E. R. Thornton, A. B. Smith, III, and R. Hirschmann. Synthesis and Binding Affinities of Novel SRIF-mimicking β-D-Glucosides Satisfying the Requirement for a π-Cloud at C1. Org. Lett. 2005, 7, 1121-1124.
S. Neelamkavil, E. Birzin, J.-J. Feng, K.-H. Chen, A. Lin, F.-C. Chen, L. Taylor, E. R. Thornton, A. B. Smith, III, and R. Hirschmann. Replacement of Phe6, Phe7, and Phe11 of D-Trp8-Somatostatin-14 with L-Pyrazinylalanine. Predicted and Observed Ef
S. Foister, L. L. Taylor, J.-J. Feng, W.-L. Chen, A. Lin, F.-C. Cheng, A. B. Smith, III, and R. F. Hirschmann. Design and Synthesis of a Potent Cystine-Free Cyclic Hexapeptide Agonist at the Human Urotensin Receptor. Org. Lett. 2006, 8, 1799-1802.
B. P. Mowery, V. Prasad, C. S. Kenesky, A. R. Angeles, L. L. Taylor, J.-J. Feng, W.-L. Chen, A. Lin, F.-C. Cheng, A. B. Smith, III, and R. Hirschmann. Catechol: A Minimal Scaffold for Non-Peptide Mimetics of the i+1 and i+2 Positions of β-turns. Ap
![]()

