Cis-regulatory elements (CREs) are critical in coordinating the regulation of gene expression. Understanding how CREs evolve represents a challenging endeavor. Here, we constructed a comprehensive single-cell atlas of CRE activity in Oryza sativa, integrating data from 104,029 nuclei representing 128 discrete cell states across nine distinct organs. We used a comparative genomic approach to analyze cell-type-specific CRE activity between Oryza sativa and four additional grass species (Zea mays, Sorghum bicolor, Panicum miliaceum, and Urochloa fusca). We revealed a complex interplay whereby accessible chromatin regions (ACRs) had different levels of conservation dependent on if these regions were broadly accessible, or cell-type specific. We found that on average epidermal ACRs were less conserved in regions of synteny compared to other cell types, potentially indicating that large amounts of regulatory evolution have taken place in the epidermal layers of these species. We found a significant overlap between cell-type-specific ACRs and conserved non-coding sequences. Finally, we identified a subset of ACRs that were overlapping the repressive histone modification H3k27me3, which were conserved across evolutionary time, implicating them as potentially critical silencer CREs. Collectively, these results highlight the dynamic evolution of cell-type-specific CRE activity using comparative genomics. Read more
Category: Publications
A rapid and sensitive, multiplex, whole mount RNA fluorescence in situ hybridization and immunohistochemistry protocol
Background
In the past few years, there has been an explosion in single-cell transcriptomics datasets, yet in vivo confirmation of these datasets is hampered in plants due to lack of robust validation methods. Likewise, modeling of plant development is hampered by paucity of spatial gene expression data. RNA fluorescence in situ hybridization (FISH) enables investigation of gene expression in the context of tissue type. Despite development of FISH methods for plants, easy and reliable whole mount FISH protocols have not yet been reported.
Results
We adapt a 3-day whole mount RNA-FISH method for plant species based on a combination of prior protocols that employs hybridization chain reaction (HCR), which amplifies the probe signal in an antibody-free manner. Our whole mount HCR RNA-FISH method shows expected spatial signals with low background for gene transcripts with known spatial expression patterns in Arabidopsis inflorescences and monocot roots. It allows simultaneous detection of three transcripts in 3D. We also show that HCR RNA-FISH can be combined with endogenous fluorescent protein detection and with our improved immunohistochemistry (IHC) protocol.
Conclusions
The whole mount HCR RNA-FISH and IHC methods allow easy investigation of 3D spatial gene expression patterns in entire plant tissues. Read more
The Role and Activity of SWI/SNF Chromatin Remodelers
SWITCH deficient SUCROSE NONFERMENTING (SWI/SNF) class chromatin remodeling complexes (CRCs) use the energy derived from ATP hydrolysis to facilitate access of proteins to the genomic DNA for transcription, replication, and DNA repair. Uniquely, SWI/SNF CRCs can both slide the histone octamer along the DNA or eject it from the DNA. Given their ability to change the chromatin status quo, SWI/SNF remodelers are critical for cell fate reprogramming with pioneer and other transcription factors, for responses to environmental challenges, and for disease prevention. Recent cryo-electron microscopy and mass spectrometry approaches have uncovered different subtypes of SWI/SNF complexes with unique properties and functions. At the same time, tethering or rapid depletion and inactivation of SWI/SNF have provided novel insight into SWI/SNF requirements for enhancer activity and into balancing chromatin compaction and accessibility in concert with Polycomb complexes. Given their importance, SWI/SNF recruitment to genomic locations by transcription factors and their biochemical activity is tightly controlled. This review focuses on recent advances in our understanding of SWI/SNF CRCs in animals and plants and discusses the multiple nuclear and biological roles of SWI/SNF CRCs and how SWI/SNF activity is altered by complex subunit composition, posttranslational modifications, and the chromatin context to support proper development and response to extrinsic cues. Read more
The regulation of chromatin configuration at AGAMOUS locus by LFR-SYD-containing complex is critical for reproductive organ development in Arabidopsis
Switch defective/sucrose non-fermentable (SWI/SNF) chromatin remodeling complexes are evolutionarily conserved, multi-subunit machinery that play vital roles in the regulation of gene expression by controlling nucleosome positioning and occupancy. However, little is known about the subunit composition of SPLAYED (SYD)-containing SWI/SNF complexes in plants. Here, we show that the Arabidopsis thaliana Leaf and Flower Related (LFR) is a subunit of SYD-containing SWI/SNF complexes. LFR interacts directly with multiple SWI/SNF subunits, including the catalytic ATPase subunit SYD, in vitro and in vivo. Phenotypic analyses of lfr-2 mutant flowers revealed that LFR is important for proper filament and pistil development, resembling the function of SYD. Transcriptome profiling revealed that LFR and SYD shared a subset of co-regulated genes. We further demonstrate that the LFR and SYD interdependently activate the transcription of AGAMOUS (AG), a C-class floral organ identity gene, by regulating the occupation of nucleosome, chromatin loop, histone modification, and Pol II enrichment on the AG locus. Furthermore, the chromosome conformation capture (3C) assay revealed that the gene loop at AG locus is negatively correlated with the AG expression level, and LFR-SYD was functional to demolish the AG chromatin loop to promote its transcription. Collectively, these results provide insight into the molecular mechanism of the Arabidopsis SYD-SWI/SNF complex in the control of higher chromatin conformation of the floral identity gene essential to plant reproductive organ development. Read more
Plant transcription factors – being in the right place with the right company. Curr Opin Plant Biol 2022
Transcriptional regulation underlies many of the growth and developmental processes that shape plants as well as their adaptation to their environment. Key to transcriptional control are transcription factors, DNA-binding proteins that serve two essential functions: to find the appropriate DNA contact sites in their target genes; and to recruit other proteins to execute transcriptional transactions. In recent years, protein structural, genomic, bioinformatic, and proteomic analyses have led to new insights into how these central functions are regulated. Here, we review new findings relating to plant transcription factor function and to their role in shaping transcription in the context of chromatin. Read more
H2A.Z contributes to trithorax activity at the AGAMOUS locus
In multicellular eukaryotes, Polycomb repression heritably silences gene expression programs not needed or detrimental for a given developmental stage or tissue (Schuettengruber et al., 2017). During cell fate reprogramming, Polycomb silencing can be overcome by the combined activity of multiple Trithorax group (TrxG) proteins (Wu et al., 2012; Liang et al., 2015; Schuettengruber et al., 2017). TrxG proteins are genetically defined as suppressors of homeotic defects caused by loss of Polycomb function and have diverse enzymatic activities (Schuettengruber et al., 2017). We used a genetic enhancer screen to identify candidate TrxG proteins and uncovered TrxG activity for components of the SWR1 chromatin remodeling complex, which deposits the histone variant H2A.Z (Deal et al., 2007; March-Diaz et al., 2008). Read more
Greenscreen: A simple method to remove artifactual signals and enrich for true peaks in genomic datasets including ChIP-seq data
Abstract
Chromatin immunoprecipitation followed by sequencing (ChIP-seq) is widely used to identify factor binding to genomic DNA and chromatin modifications. ChIP-seq data analysis is affected by genomic regions that generate ultra-high artifactual signals. To remove these signals from ChIP-seq data, the Encyclopedia of DNA Elements (ENCODE) project developed comprehensive sets of regions defined by low mappability and ultra-high signals called blacklists for human, mouse (Mus musculus), nematode (Caenorhabditis elegans), and fruit fly (Drosophila melanogaster). However, blacklists are not currently available for many model and nonmodel species. Here, we describe an alternative approach for removing false-positive peaks called greenscreen. Greenscreen is easy to implement, requires few input samples, and uses analysis tools frequently employed for ChIP-seq. Greenscreen removes artifactual signals as effectively as blacklists in Arabidopsis thaliana and human ChIP-seq dataset while covering less of the genome and dramatically improves ChIP-seq peak calling and downstream analyses. Greenscreen filtering reveals true factor binding overlap and occupancy changes in different genetic backgrounds or tissues. Because it is effective with as few as two inputs, greenscreen is readily adaptable for use in any species or genome build. Although developed for ChIP-seq, greenscreen also identifies artifactual signals from other genomic datasets including Cleavage Under Targets and Release Using Nuclease. We present an improved ChIP-seq pipeline incorporating greenscreen that detects more true peaks than other methods. Read more
New review on Polycomb Repressive Complex 2 published with Dr. Tomasz Bieluszewski and Dr. Jun Xiao
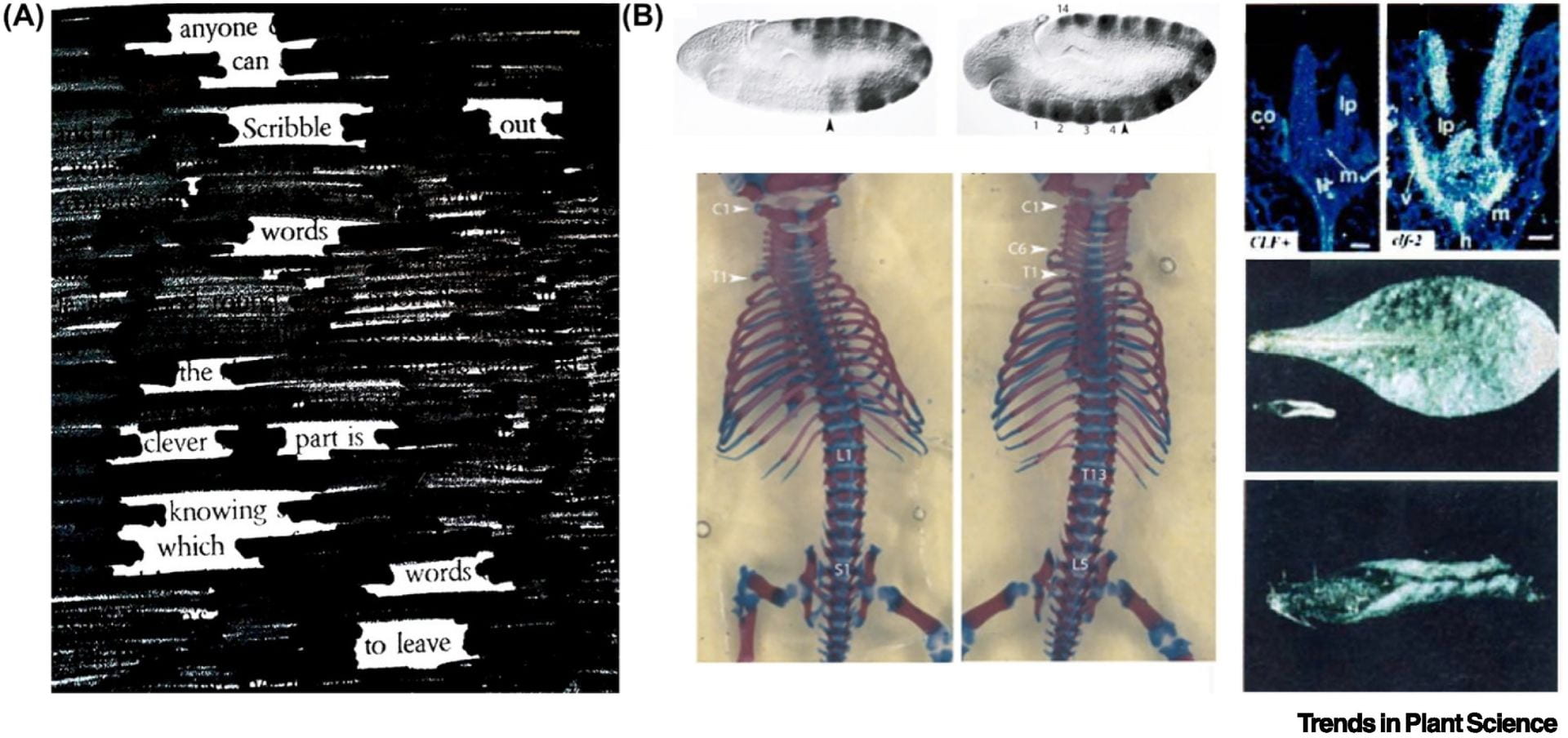
This review covers comparative analysis of PRC2 structure and function in animals and plants:
First ever ‘pioneer’ factor found in plants enables cells to change their fate

The Wagner Lab’s most recent publication uncovers the role of a protein called “LFY” in Arabidopsis chromatin regulation. A news release on the publication was covered by the American Association for the Advancement of Science (AAAS). To see the article click here.