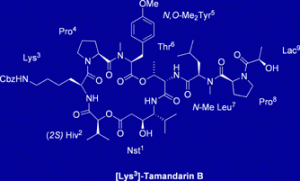
Synthesis of Lys3 Tamandarin B
The Tamandarin and Didemnin family of natural products have a vast array of biological activity and are potential therapeutic agents, yet their mechanism of action is unknown. This analogue will be used in an affinity chromatography study to help determine the mechanism of action.
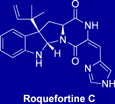
Synthesis of Roquefortine C
Roquefortine C is a natural product isolated from Penicillium roquefortii, a fungus which is widely used in food industry. Neurotoxicity of roquefortine C was reported by several groups, but the data were inconsistent. The mechanism underlying its toxicity and metabolism are still unknown. To solve the long existing food safety concerns related to roquefortine C, we recently completed the first total synthesis of this compound.
Synthesis of Trichodermamides A and B Trichodermamides A and B are two modified dipeptides possessing a novel 4(H)-5,6-dihydro-1,2-oxazine functionality incorporated into a highly functionalized cyclohexene ring with four contiguous stereocenters. Trichodermamide B displayed significant in vitro cytotoxicity against HCT-116 human colon carcinoma. We are currently pursuing the total synthesis of the trichodermamides.
Synthesis of Ustiloxins and Phomopsins
The ustiloxin and phomopsin families of natural products are heterodetic peptides, which are potent microtubule depolymerizers. Both ustiloxins and phomopsins have a similar 13-memebered macrolactam, and have a unique tertiary alkyl aryl ether linkage. The tertiary alkyl aryl ether linkage has been constructed by an aziridine ring opening reaction in a highly stereoselective and regioselective manner.