Biophysics and Physiology of Intracellular Organelles
A hallmark of eukaryotic cells is the separation of cellular functions among membrane-enclosed organelles such as nuclei, mitochondria, endoplasmic reticulum, endosomes and lysosomes. Intracellular membranes constitute > 90% of total cell membrane, but, in contrast to those of plasma membrane, their biophysical properties have been little understood. We have recently started to apply current-clamping electrophysiological recordings to record the organelle membrane potentials and their regulation, and voltage-clamp recordings to reveal the major endosomal and lysosomal conductances (Cang et al. 2014). We then used functional screening and candidate approaches to identify the genes coding the conductances. The major goals are to define the organelle’s biophysical properties, to reveal the underlying proteins, and to uncover their function in organismal physiology.
(Movie: Endosomes labeled with RFP-fused Rab7 transfected in a cultured hippocampal neuron. Movie recorded by Youngjun Seo at Ren lab.)
Membrane potentials of endosomes and lysosomes
Using current-clamp recordings, we have directly recorded membrane potentials (ΔΨ) of endosomes and lysosomes from various cell types such as fibroblasts, macrophages, glia, cardiac myocytes, kidney cells and neurons (Cang et al. 2014). Like plasma membrane potential Vm, ΔΨ is influenced by K+ and Na+. In lysosomes, in which luminal pH is highly acidic (pH ~ 4.7), H+ also makes large contribution to the resting ΔΨ. A surprising finding was that some lysosomes such as those from cardiac myocytes are electrically excitable: a depolarizing stimulus above threshold triggers an ‘action potential–like’ long-lasting depolarization and sometimes leads to bistability of ΔΨ. The function of such ΔΨ excitability is currently unknown.


Patch-clamp recording from endosomes and lysosomes. Upper left, endosomes are enlarged and marked by transfecting mCherry-Rab5-Q79L. A glass pipette is used to slice the cell membrane and release the organelle (indicated by arrow). Upper right, whole-organelle patch clamp configuration. Lower, examples of current-clamp recordings from a lysosome liberated from a beating cardiac myocyte. Current injections above threshold trigger long-lasting depolarization spikes. The spike-generation is dependent on the non-inactivating voltage-gated Na+ channel TPC1.
(Movie: patch clamp recording of lysosomes. An individual lysosome is dissected out with a glass pipette for patch clamp recording. Movie recorded by Chunlei Cang at Ren lab.)
Ionic conductances of endosomes and lysosomes
We have used voltage-clamp to systematically “catalog” the ionic conductances in the organelles, and have detected several major K+, Na+, Cl– and H+ conductances (Cang et al. 2014). The organelle membranes are highly permeable to H+ (PH/PK = 7,185 ± 720 in macrophage lysosomes), and, like plasma membrane, are generally more permeable to K+ than to Na+ at “resting”. The relative permeability of Na+ to K+ (PNa/PK), however, can be drastically increased by >30 fold upon physiologically stimuli such as increases in PI(3,5)P2 concentration, decreases in cytoplasmic ATP concentration or alkalinization of organelle lumen (Cang et al. 2014).
The readily detectable ionic conductances of lysosomes under voltage clamp are those of K+, Na+, Cl– and H+. The Ca2+ conductance is very small, but there are clearly Ca2+-permeable channels on the lysosome.
Ion channels of endosomes and lysosomes
The major ionic conductances have now been molecularly defined in the past several years. We have used expression cloning and candidate approaches to identify the K+ can Na+ channels we detected with patch clamp recordings.
Endosomal/lysosomal K+ channel KEL (TMEM175)
The major K+ channel in endosomes and lysosomes is a voltage-independent “leak-like” K+ channel (KEL). Using a proteomic-based candidate gene screening, we found that KEL is formed by TMEM175. Unlike the canonical plasma membrane K+ channels, TMEM175 has a predicted 2x6TM structure and doesn’t appear to have a P-loop or a GYG motif-containing channel filter. TMEM175 homologs are also present in archaea and bacteria. An area of research is to define the structural basis for TMEM175’s K+ selectivity.
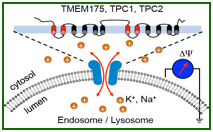
Endosomal/lysosomal K+ channel (TMEM175, voltage-independent) and Na+ channels (TPC1, voltage-dependent; TPC2, voltage-independent)
Endosomal/lysosomal voltage-gated Na+ channel lysoNaV (TPC1)
Endosomes/lysosomes have their own voltage-gated Na+ channel (lysoNaV) (Cang et al. 2014). The channel is formed by TPC1, a member of the two-repeat (two-pore) channels. TPCs share sequence similarity with NaVs and CaVs but have a 2x6TM structure instead of the 4x6TM in NaVs and CaVs (see table). Unlike plasma membrane NaVs, TPC1 has no inactivation. Under physiological conditions of lumen pH of ~ pH4.7, the channel is activated at high voltages (> 0 mV), but the activation threshold can be drastically shifted toward lower voltages by luminal alkalinization (~ 60 mV shift of voltage-dependence by a 1 unit of pH change). This high pH sensitivity can perhaps be used by the organelles to keep pH stability (see a model).
Like its plasma membrane counterpart voltage-gated Na+ channels, TPC1 is required for the generation of long-lasting action potential-like spikes in the lysosomes. The function of the spikes is totally unknown.
Endosomal/lysosomal voltage-independent Na+ channel (TPC2)
Lysosomes also have a voltage-independent, “leak-like” Na+ channel that presumably helps to determine the “resting” membrane potential. The channel is formed by the TPC2 protein. Unlike TPC1, TPC2 has little voltage-dependence.
Both TPC1 and TPC2 are primarily Na+-selective. Studies from other investigators suggest that the channels also conduct Ca2+ under certain conditions.
The TPC ion channel family has a third member TPC3, which is present in some animals such as zebrafish, frogs and birds, but not in humans. TPC3 forms a plasma membrane high voltage-activated non-inactivating Na+ channel that can support the generation of ultra-long action potentials (ulAPs) lasting many seconds to minutes. Ultra-long APs have been recorded from marine animals and, in the eggs of some species, have been proposed to be used as a barrier to polyspermy fertilization. Why some animals have acquired TPC3 and the potential uses of ultra-long action potentials remain to be discovered.
Regulation of lysosomal ion channels by intracellular and extracellular factors
Lysosomal ion channels are regulated by both intracellular and extracellular factors. For example, the TPC1 and TPC2 channels can be regulated by the luminal pH inside the lysosome (TPC1), the voltage across them organelle membrane (TPC1), membrane lipids (TPC1, TPC2 and TMEM175) and cytosolic ATP (TPC1 and TPC2).
Lysosomal ion channels can be regulated by extracellular factors such as circulating amino acids. In the presence of extracellular amino acids, mTOR (Mechanistic Target of Rapamycin) translocates onto lysosomal surface and inhibits TPC1 and TPC2. Upon starvation of amino acids, mTOR dissociates from lysosomes and TPCs open.
Physiological functions of organelle ion channels
The functions of lysosomal/endosomal ion channels are largely unknown. To define the functions, we generate mutant mice with homologous recombination and/or the CRISPR/Cas9 genome-editing techniques. Both TPC and TMEM175 mutant cells have compromised stability in lysosomal pH, especially during nutrient starvation, suggesting roles of the channels in supplying counter-ions in the establishment and maintenance of acidic luminal pH of the organelle.
A unique feature of lysosomes and endosomes is their high motility within the cell. In neurons, for examples, they travel up to a meter along the axons. A current area of research is to investigate the roles of ion channels and associated membrane potential changes in organelle motility and signaling. We found that lysosomes use Na+ channels as a multimodal sensor to monitor the luminal pH, the voltage gradient across organelle membrane, the metabolic state (intracellular [ATP]) and the availability of nutrients (amino acids in particular) outside the cell. The nutrient- and ATP-sensing ability of lysosomal Na+ channels requires mTOR, a kinase that accumulates onto lysosomal surfaces in nutrient-replete cells but translocates away upon nutrient depletion. TPC knockout mice have compromised amino acid metabolism and reduced physical endurance. In TMEM175 knockout cells, the lysosomes are depolarized and have abnormal fusion with autophagosomes. Future studies will investigate the channels’ function in physiological and pathophysiological processes such as lysosomal digestion/storage/export, immune defense, metabolism and neuronal degeneration.
Neurodegenerative diseases and lysosomal ion channels
(under construction)
|
|
Key References:
Cang, C., Aranda, K., Seo, Y.-J., Gasnier, B. and Ren, D. (2015) TMEM175 is an organelle K+ channel regulating lysosomal function. Cell 162:1101-12
Xu, H. and Ren, D. (2015) Lysosomal Physiology. Annu. Rev. Physiol. 77:57-80
Cang, C., Bekele, B. and Ren, D. (2014) The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nature Chem. Biol. 10: 463-469
Cang, C., Aranda, K. and Ren, D. (2014) A non-inactivating high-voltage-activated two-pore Na+ channel that supports ultra-long action potentials and membrane bistability. Nature Commun. 5: 5015
Cang, C.*, Zhou, Y.*, Navarro, B., Aranda, K., Shi, L., Battaglia-Hsu, S., Nissim, I., Clapham, D. and Ren, D. (2013) mTOR regulates lysosomal ATP-sensitive two-Pore Na+ channels to adapt to metabolic state. Cell 152: 778-790
Wang, X., Zhang, X., Dong, X.-P., Samie, M., Li, X., Cheng, X., Goschka, A., Shen, D., Zhou, Y., Harlow, J., Zhu, M.-X., Clapham, D.E., Ren, D., and Xu, H. (2012) TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell 151:372-383
